Resources
Latest ICH E6(R3) GCP Update Explained
Here are 6 key updates from ICH E6(R2) to E6(R3)

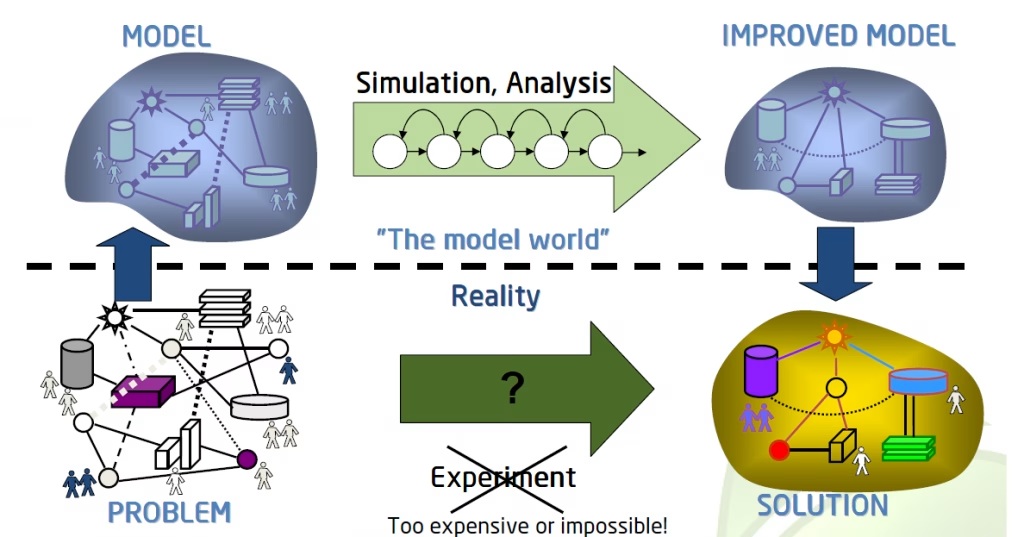
10 Reasons to Implement Modelling and Simulations Into Your Drug Development Program
Here are 10 key reasons how you can benefit from modelling and simulations:
Project Optimus in oncology drug development
It is now a requirement for all sponsors to implement Project Optimus into their oncology drug development programs for dose optimisation.

Cannabis-based products for medical use in humans
The UK regulatory agency, MHRA, has published a guidance on the supply, manufacture, importation and distribution of unlicensed cannabis-based products for medicinal use in humans.

Clinical pharmacology in dermatology drug development
Pharmacokinetic Considerations for Topical Medications